Blogs
March 11, 2022To what extent is fuel cell commercialisation inhibited
With the world population (7.7 billion today) expected to continue its explosive growth, coupled with industrial expansions, the world energy consumption is expected to grow by nearly 45% by 2050. The Paris agreement is implemented to limit global warming preferably to 1.5 degrees Celsius, compared to pre-industrial levels, providing energy in an environmentally friendly manner is a critical issue facing the planet. In COP26 the urgency of overcoming this collective challenge was emphasised by world leaders, who have clear intentions of accelerating action by 2030. Consequently, the growing awareness is pushing for the implementation of alternative sustainable technologies. Among them, fuel cells, specifically low temperature (<100 ˚C) polymer electrolyte membrane fuel cells (PEMFC) are looking promising in helping provide cleaner energy due to their wide range of applications within transportation, stationary and portable power generations. A PEMFC is currently utilised on a commercial scale for FCVs. This blog will explore the current feasibility of the fuel cells with focus on the electrocatalyst and their future potential for large scale implementations.
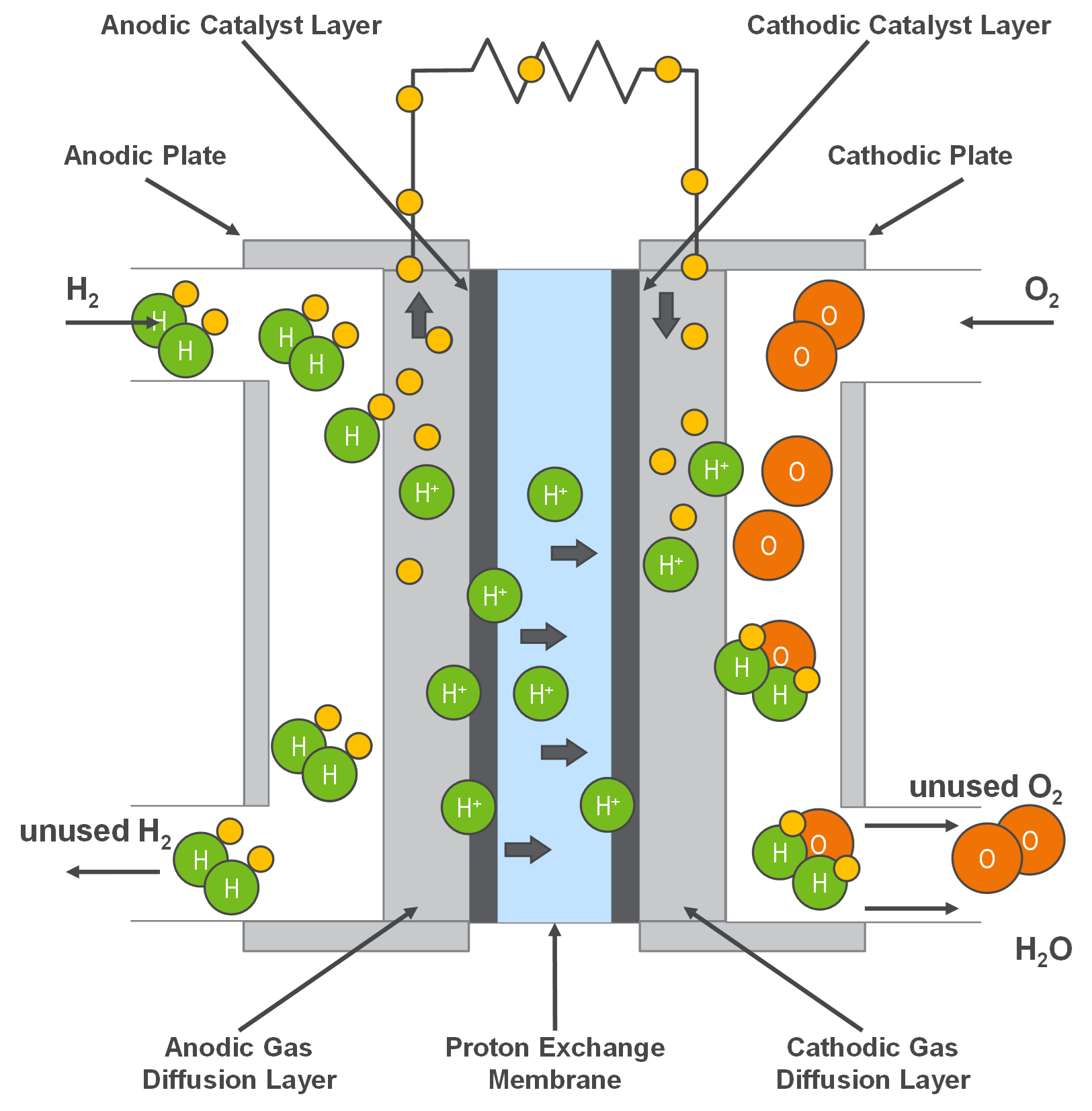
A PEMFC currently uses a water-based, acidic polymer membrane as its electrolyte, with platinum-based catalysts sandwiched between the electrodes and membrane. There is also focus on replacing electrodes with a more sustainable material, although most have not been successful in achieving the same performances at this stage. Due to relatively low operating temperatures (< 100 ˚C) and the use of precious metal-based electrodes these cells must operate on pure hydrogen. Due to the wide range of applications as mentioned above, PEMFCS have received intense attention which has led to the development of promising electrochemical-generating devices. These alternative sources provide the possibility of receiving energy from hydrogen.
The image above shows the flow of hydrogen to the anode where electrons are separated from the protons on the surface of a platinum-based catalyst. The protons then pass through the membrane to the cathode while electrons travel in an external circuit generating an electrical output. On the cathode side the catalyst combines protons and electrons with oxygen to produce water. Water is the only waste product (and heat), and the oxygen can either be provided in a purified form or extracted at the electrode directly from the air.
PEMFCs are expected to play an important role in making the transportation industry more sustainable. Life cycle analysis have also been conducted to show the benefits of using a fuel cell vehicle compared to gasoline and battery electric vehicles.
Currently Pt-based electrocatalyst are implemented in commercial fuel cells to facilitate the electrochemical reactions. At the anode, the reactions possess simple and fast reactions kinetics, enabling relatively low loadings of platinum (Pt). Although, at the cathode oxygen reduction reaction (ORR) has sluggish kinetics and therefore requires significant quantities (~4-6 times higher than anode) of Pt-based electrocatalysts which makes fuel cells too expensive for large scale implementations. Pt possess high ORR activity because of its desirable binding energy with O and OH. Pt/C catalyst is a common commercial fuel cell cathode electrocatalyst where the performance depends on the crystallization, morphology, shape, and size of the platinum.
There have been many attempts to reduce the Pt loadings by providing novel catalysts supports such as carbon nanotubes and single-walled carbon nanotubes. Although there have been promising attempts to develop alternatives (e.g., Pt-Metal alloy) which are comparable to the current state-of-the-art Pt/C due to the low recycling of Pt and increasing future demand the use of Pt is likely to case long term issues. This along with the scarcity of the metal can make the use of Pt infeasible for large scale applications in the long term. Previous studies have shown that the catalyst makes up the largest single component (>40%) of the PEMFC stack cost at high volume (500 000 vehicles). Additionally, price volatility of Pt impacts the fuel cell costs and a high volatility in the pricing of the fuel cells make it difficult to commercialise. It is also observed that South Africa produces the bulk of the worlds Pt supply (68%), this dependency on the nation is unfavourable due to the fragility of global supply networks and trade agreements. These issues with Pt-based catalysts indicate as to why the elimination of platinum group metal (PGM) catalysts is desirable for the future of the fuel cell industry.
There is great interest in M-N-C (metal coupled with N-doped carbon) catalysts and the use of non-precious metals due to their low cost, high abundance, and being readily accessible. Many research organisations (ElectroCat) and commercial companies (Pajarito Powders & Nasshinbo holdings) are involved in the development of M-N-C and other PGM-free catalysts. Many of these have demonstrated promising ORR activities at lab scale. Among those Fe-N-C has been commercialised by Pajarito Powders which has displayed ORR activity that is comparable to the commercial Pt/C catalyst.
Previously, key players within the industry have conducted a techno-economic assessment to estimate the production costs at multiple rates of annual productions. The preliminary results for a Polyaniline (PANI)-Fe-C synthesis showed very low cost of catalyst per mass of material ~US$73/kg PANI vs ~US$41,000/kg Pt-based. Although the PANI-Fe-C must significantly increase its power density to achieve a lower stack cost than the Pt-catalysed stacks. This is because the use of this catalyst under current conditions will require much higher loadings increasing the mass of catalyst powder required. Improving performance of these PGM-free catalysts can help provide a promising economically and environmentally feasible alternative, although this is only possible if they are scalable and achieve similar performances to the Pt/C catalyst.
Environmentally, the elimination of Pt can be extremely advantageous especially if this is replaced by an abundant metal like iron (Fe). For instance, studies have shown that only 0.75 g of Pt in the manufacturing phase contributes to 60% of total environmental impacts for the manufacturing phase of a 1-KW PEMFC system. When considering the recycling of Pt, ~60% of the environmental impacts can be reduced if ~90% of the Pt is recycled. However, to date even the most efficient lab scale Pt recovery process only recovers ~75% of the Pt from a PEMFC.
To conclude, the use of the current Pt/C electrocatalyst in fuel cells makes it infeasible for large scale productions. Even though, there are many technological advances and alternatives to the electrocatalysts, currently they cannot replace the Pt/C due to lower performance making them economically infeasible. The intense research in this sector by various organisations and companies is likely to yield a feasible catalyst within the next 5 – 10 years which can allow the large-scale implementations of PEMFCs.
The Author
Jinil Pandya, Analyst
About Us - NexantECA, the Energy and Chemicals Advisory company is the leading advisor to the energy, refining, and chemical industries. Our clientele ranges from major oil and chemical companies, governments, investors, and financial institutions to regulators, development agencies, and law firms. Using a combination of business and technical expertise, with deep and broad understanding of markets, technologies and economics, NexantECA provides solutions that our clients have relied upon for over 50 years.